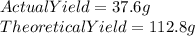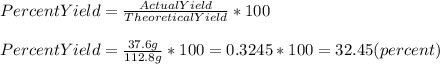Chemistry, 27.06.2019 08:30, Janznznz4012
If the actual yield of a reaction is 37.6 g while the theoretical yield is 112.8 g what is the percent yield

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2
Do you know the correct answer?
If the actual yield of a reaction is 37.6 g while the theoretical yield is 112.8 g what is the perce...
Questions in other subjects:


Physics, 28.02.2020 23:15



Spanish, 28.02.2020 23:15

Mathematics, 28.02.2020 23:16

Business, 28.02.2020 23:16

Mathematics, 28.02.2020 23:16