
Chemistry, 28.06.2019 03:00, aidentrooper8629
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric acid. if the percent yield of the reaction was 82.15%, what was the actual amount of calcium chloride formed? caco3 + hcl → cacl2 + co2 + h2o 105.3 grams 101.1 grams 95.6 grams 86.5 grams

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
Do you know the correct answer?
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric ac...
Questions in other subjects:

Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30



Mathematics, 03.02.2021 21:30




Mathematics, 03.02.2021 21:30


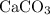 ?
?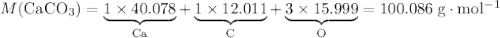 .
.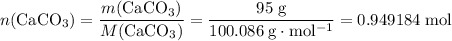 .
.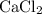 will be produced?
will be produced?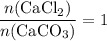 .
.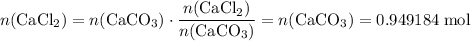 .
.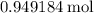 of
of 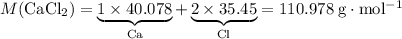 .
.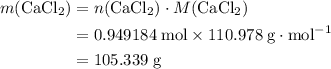 .
. .
. .
.





