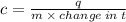When 45 g of an alloy, at 25°c, are dropped into 100.0g of water, the alloy absorbs 956j of heat. if the temperature of the alloy is 37°c, what is its specific heat? a. 0.423 cal/g°c b. 1.77 cal/g°c c. 9.88 cal/g°c d. 48.8 cal/g°c try and explain with step by step or show work,

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
Do you know the correct answer?
When 45 g of an alloy, at 25°c, are dropped into 100.0g of water, the alloy absorbs 956j of heat. if...
Questions in other subjects:

Chemistry, 09.10.2019 06:30





Social Studies, 09.10.2019 06:30

Mathematics, 09.10.2019 06:30


Mathematics, 09.10.2019 06:30

Mathematics, 09.10.2019 06:30