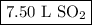Chemistry, 09.10.2019 10:50, dmead22284
When 7.50 l of sulfur trioxide are produced by the reaction of sulfur dioxide in an excess of oxygen at standard temperature and pressure, how many liters of sulfur dioxide were used? 2so2 (g) + o2 (g) yields 2so3 (g)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
Do you know the correct answer?
When 7.50 l of sulfur trioxide are produced by the reaction of sulfur dioxide in an excess of oxygen...
Questions in other subjects:








Computers and Technology, 23.10.2019 03:00