
Chemistry, 29.06.2019 12:10, claftonaustin846
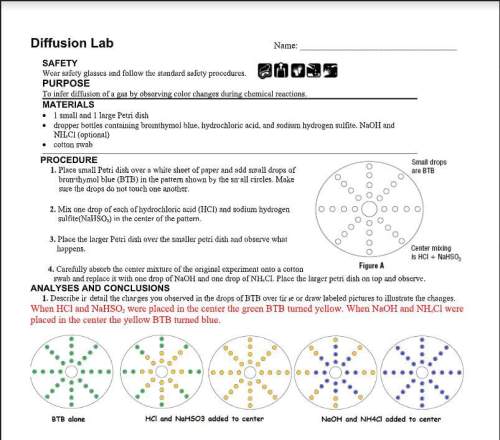
2. the btb changed even though you added nothing to it. if the mixture in the center circle produced a gas, would this explain the change in the drops of btb? use kinetic theory to explain your answer.3. why were you asked to place the drops of btb further and further from the center (where the chemical reaction took place)? 4. imagine a room filled with air. in the center of this is a vial of an unknown gas with the same density as air. now the vial isbroken and the unknown gas escapes into the air. after twenty minutes are there more, less or the same number of unknown gasparticles near the vial as there were when it was first broken? why? 5. many of the behaviors observed in this lab were due to gas diffusion. define diffusion in your own words.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
2. the btb changed even though you added nothing to it. if the mixture in the center circle produced...
Questions in other subjects:





Arts, 22.04.2021 18:10

Mathematics, 22.04.2021 18:10


Chemistry, 22.04.2021 18:10







