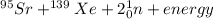Chemistry, 29.06.2019 14:10, phsycotic121
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are present before the reaction and the atoms on the right of the equal sign are produced after the reaction. model 1: atom 1 + atom 2 = atom 3 + energymodel 2: atom 4 = atom 5 + atom 6 + energywhich of these statements is most likely correct about the two models? both models show reactions which use up energy in the sun. both models show reactions which produce energy in the sun. model 1 shows reactions in the sun and model 2 shows reactions in the nuclear power plants. model 1 shows reactions in the nuclear power plants and model 2 shows reactions in the sun.

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
Do you know the correct answer?
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are pr...
Questions in other subjects:











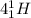 →
→ 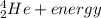 + some other particles
+ some other particles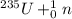 →
→