
Chemistry, 29.06.2019 16:40, savannahvargas512
Achemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. the vessel is a stainless-steel cylinder that measures 17.0cm wide and 20.4cm high. the maximum safe pressure inside the vessel has been measured to be 2.20mpa. for a certain reaction the vessel may contain up to 0.0985kg of boron trifluoride gas. calculate the maximum safe operating temperature the engineer should recommend for this reaction. write your answer in degrees celsius. round your answer to significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 14:30, jenorajordan5387
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1
Do you know the correct answer?
Achemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reac...
Questions in other subjects:


Social Studies, 30.07.2019 17:30

Social Studies, 30.07.2019 17:30




History, 30.07.2019 17:30

History, 30.07.2019 17:30

History, 30.07.2019 17:30

History, 30.07.2019 17:30


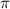
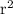 h
h 17
17 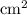 = 4.630
= 4.630 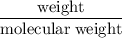 moles of boron trifluoride =
moles of boron trifluoride = 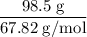 moles of boron trifluoride = 1.452 molPV = nRTT =
moles of boron trifluoride = 1.452 molPV = nRTT = 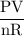 T =
T = 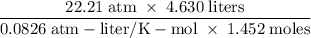 = 863 K= 863 -
= 863 K= 863 - 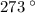 C=
C= 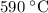 .
.



