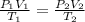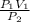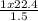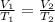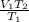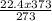Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combined gas law. c. if the pressure increases to 1.50 atm and the temperature doesn’t change, calculate the new volume. d. if the temperature increases to 100.0 ˚c and the pressure doesn’t change, calculate the new volume.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 02:00, ItzAquaZ1449
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
Do you know the correct answer?
Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combi...
Questions in other subjects:





Physics, 27.10.2020 07:20

English, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20

Mathematics, 27.10.2020 07:20