
Chemistry, 31.12.2019 15:31, jackfrost5
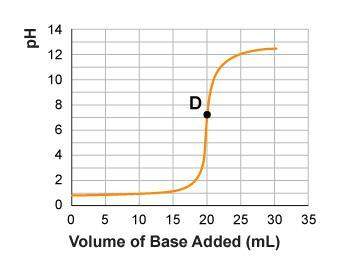
Some students performed a titration between 20.0 ml of 0.5 m hydrochloric acid and 1.0 m potassium hydroxide solution. the students collected data and plotted the graph below. which statement correctly explains the reaction at point d?
option a) all hydroxide ions have reacted. there is no excess of hydroxide ions at this point.
option b) the volume of base that has been added is equal to the volume of acid in the flask; this in balancing the ions present, making the ph of the solution neutral.
option c) all hydrogen ions and all hydroxide ions have reacted to produce water, and so neither ion remains free in solution.
option d) there are extra hydrogen ions in solution. as the base is added, the ph increases exponentially.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
Do you know the correct answer?
Some students performed a titration between 20.0 ml of 0.5 m hydrochloric acid and 1.0 m potassium h...
Questions in other subjects:




Mathematics, 12.10.2020 05:01

Mathematics, 12.10.2020 05:01





English, 12.10.2020 06:01






