
Chemistry, 22.09.2019 18:30, lillyd2873
Asample of a compound analyzed in a chemistry laboratory consists of 5.34 g of carbon, 0.42 g of hydrogen, and 47.08 g of chlorine. what is the percent composition of this compound?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
Do you know the correct answer?
Asample of a compound analyzed in a chemistry laboratory consists of 5.34 g of carbon, 0.42 g of hyd...
Questions in other subjects:

Physics, 19.08.2019 05:30

Social Studies, 19.08.2019 05:30

Mathematics, 19.08.2019 05:30

Social Studies, 19.08.2019 05:30

Biology, 19.08.2019 05:30

Mathematics, 19.08.2019 05:30

History, 19.08.2019 05:30

Health, 19.08.2019 05:30



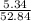 × 100 = 10.1 %
× 100 = 10.1 %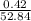 × 100 = 0.8 %
× 100 = 0.8 %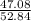 × 100 = 89.1 %
× 100 = 89.1 %



