Pls will mark brainliest. need answered now
what is the mass of a sample of nh3 containi...

Chemistry, 22.10.2019 06:00, electronia
Pls will mark brainliest. need answered now
what is the mass of a sample of nh3 containing 3.80 × 1024 molecules of nh3?
74.8
89.4
101
107

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sairaanwar67
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2


Chemistry, 22.06.2019 06:00, VamPL
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl +4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 27.07.2019 13:30


Chemistry, 27.07.2019 13:30

Computers and Technology, 27.07.2019 13:30


Chemistry, 27.07.2019 13:30


Social Studies, 27.07.2019 13:30


 contain
contain  molecules of
molecules of 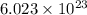 molecules of
molecules of 




