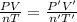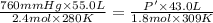Chemistry, 24.08.2019 09:50, greenbyron88
Initially, a 55.0 liter compressible container, holding 2.4 moles of a gas, exerts a pressure of 760 millimeters of mercury at a temperature of 280 kelvin. what is the pressure when the container is compressed to 43.0 liters, the moles of gas reduces to 1.8 moles, and the temperature changes to 36 degrees celsius?
93.7 mm hg
492 mm hg
740 mm hg
805 mm hg

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1


Chemistry, 23.06.2019 09:30, crawford184232323234
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2

Chemistry, 23.06.2019 10:50, broyochey1
Which compound should undergo substitution of the bromine by phenolate anion? draw the structure of the organic product?
Answers: 1
Do you know the correct answer?
Initially, a 55.0 liter compressible container, holding 2.4 moles of a gas, exerts a pressure of 760...
Questions in other subjects:

Mathematics, 22.08.2019 18:00

History, 22.08.2019 18:00

Chemistry, 22.08.2019 18:00


Mathematics, 22.08.2019 18:00



History, 22.08.2019 18:00



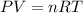
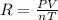 ...(1)
...(1)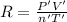 ..(2)
..(2)