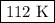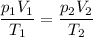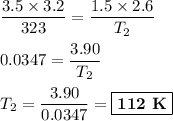Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm a...

Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm and a temperature of 323 k, what will be the temperature of the gas if i decrease the volume of the gas to 2.6 l and decrease the pressure to 1.5
answer all parts below for full credit:
a) what are the knows in this problem
b) what is the problem asking you to find
c) which gas law is the best law for finding the answer to this problem?
d) use the gas law that you indicated in part c above and find the unknown value(be sure to show all of your work)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Do you know the correct answer?
Questions in other subjects:






Mathematics, 11.10.2019 00:00

Geography, 11.10.2019 00:10


History, 11.10.2019 00:10

Geography, 11.10.2019 00:10