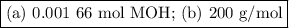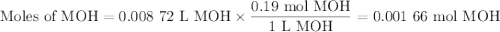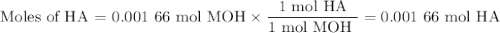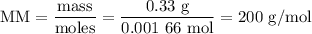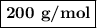Chemistry, 20.10.2019 06:50, goldenarrow
Using the concentration of the base and the volume of the base used, calculate the moles of the base used in the titration. then, using the mass of the acid, determine the molar mass of the acid.
data:
concentration of the base(naoh)= 0.19 m
volume of the base used= 8.72 ml
mass of the acid(unknown)= 0.33 g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3


Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
Do you know the correct answer?
Using the concentration of the base and the volume of the base used, calculate the moles of the base...
Questions in other subjects:






Computers and Technology, 16.12.2020 20:10

Mathematics, 16.12.2020 20:10


Mathematics, 16.12.2020 20:10