Chemistry, 31.01.2020 13:56, sierravick123owr441
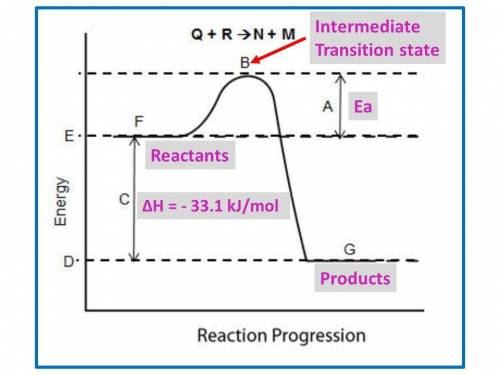
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs= 63.02 j/(mol·k).
i. draw a possible potential energy diagram of the reaction. label the enthalpy of the reaction.
ii. is the reaction endothermic or exothermic? explain your answer. (2 points)
iii. what is the gibbs free energy of the reaction at 25°c?
iv. is the reaction spontaneous or nonspontaneous at 25°c? explain your answer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Do you know the correct answer?
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs...
Questions in other subjects:


Health, 15.01.2021 20:40

Mathematics, 15.01.2021 20:40

Health, 15.01.2021 20:40

Mathematics, 15.01.2021 20:40


Spanish, 15.01.2021 20:40

Physics, 15.01.2021 20:40