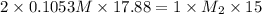Chemistry, 25.11.2019 23:31, josephsky420
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 solution to reach the equivalence point in a titration. what is the concentration of the naoh solution?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
Do you know the correct answer?
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 s...
Questions in other subjects:


Chemistry, 19.01.2021 04:00

Mathematics, 19.01.2021 04:00


Social Studies, 19.01.2021 04:00




Mathematics, 19.01.2021 04:10


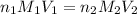
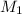 = molarity of
= molarity of 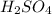 solution = 0.1053 M
solution = 0.1053 M
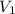 = volume of
= volume of 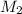 = molarity of
= molarity of 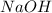 solution = 0.46 M
solution = 0.46 M
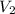 = volume of
= volume of 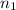 = valency of
= valency of 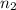 = valency of
= valency of