
Chemistry, 04.02.2020 20:45, tyliyahmiles99
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) + 7o2 (g) > 4co2 (g) +6h2o (g)
b) 2pbo (s) + pbo2 (s) > pb3o4 (s)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1
Do you know the correct answer?
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) +...
a) 2c2h6 (g) +...
Questions in other subjects:



Mathematics, 16.07.2019 00:50

Mathematics, 16.07.2019 00:50

Physics, 16.07.2019 00:50


English, 16.07.2019 00:50

Mathematics, 16.07.2019 00:50

Chemistry, 16.07.2019 00:50


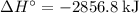 per mole reaction.
per mole reaction. per mole reaction.
per mole reaction.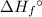 of a substance?
of a substance? 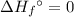 for the most stable allotrope of each element under standard conditions. For example, oxygen
for the most stable allotrope of each element under standard conditions. For example, oxygen 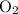 (not ozone
(not ozone 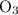 ) is the most stable allotrope of oxygen. Also, under STP
) is the most stable allotrope of oxygen. Also, under STP 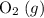 from itself does not involve any chemical or physical change. As a result,
from itself does not involve any chemical or physical change. As a result, 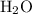 in particular) and the sign of the enthalpy changes.
in particular) and the sign of the enthalpy changes.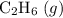 :
: 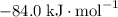 ;
;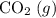 :
: 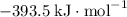 ;
;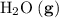 :
: 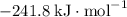 ;
;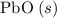 :
: 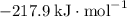 ;
;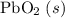 :
: 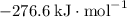 ;
;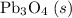 :
: 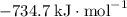
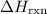 (or simply
(or simply 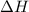 from enthalpies of formation?
from enthalpies of formation?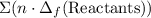 to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum
to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum 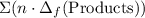 to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes.
to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes. 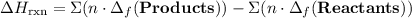 .
. 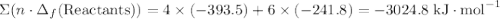 ;
;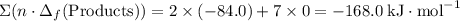 ;
;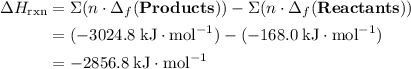 .
.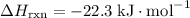 .
.



