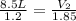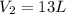Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
A8.5-liter sample of a gas at 2.0 atm and 300.0 k has 1.2 moles of the gas. if 0.65 mole of the gas...
Questions in other subjects:

Biology, 25.09.2019 16:40

History, 25.09.2019 16:40

Chemistry, 25.09.2019 16:40

Mathematics, 25.09.2019 16:40


Mathematics, 25.09.2019 16:40

Mathematics, 25.09.2019 16:40



English, 25.09.2019 16:40


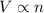 (At constant temperature and pressure)
(At constant temperature and pressure) 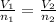
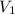 = initial volume of gas = 8.5 L
= initial volume of gas = 8.5 L
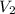 = final volume of gas = ?
= final volume of gas = ?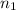 = initial moles of gas = 1.2 moles
= initial moles of gas = 1.2 moles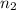 = final moles of gas = 1.2 +0.65 = 1.85 moles
= final moles of gas = 1.2 +0.65 = 1.85 moles