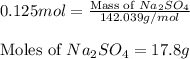Chemistry, 30.01.2020 17:02, xXFLUFFYXx
The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4 → na2so4 + h2o
how many grams of na2so4 are produced from 10.0 grams of naoh?
(molar mass of na = 22.989 g/mol, o = 15.999 g/mol, h = 1.008 g/mol, s = 32.065 g/mol)
17.8 grams
19.2 grams
35.5 grams
38.5 grams

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
Do you know the correct answer?
The following reaction shows sodium hydroxide reacting with sulfuric acid.
naoh + h2so4...
naoh + h2so4...
Questions in other subjects:

Mathematics, 22.03.2021 02:00

English, 22.03.2021 02:00



History, 22.03.2021 02:00


Spanish, 22.03.2021 02:00

Mathematics, 22.03.2021 02:00


Mathematics, 22.03.2021 02:10


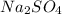 produced will be 17.8 grams.
produced will be 17.8 grams. 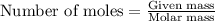 ....(1)
....(1)![NaOH=[(1\times 22.989)+(1\times 15.999)+(1\times 1.008)]g/mol=39.996g/mol](/tpl/images/0486/6119/380bc.png)
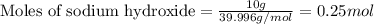
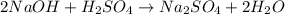
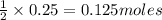 of sodium sulfate.
of sodium sulfate.![Na_2SO_4=[(2\times 22.989)+(1\times 32.065)+(4\times 15.999)]g/mol=142.039g/mol](/tpl/images/0486/6119/878b0.png)