

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Do you know the correct answer?
The reaction a + 2b + c -- > d + 2e is first order in reactant a, first order in b, and second or...
Questions in other subjects:

Biology, 02.08.2019 13:30


Mathematics, 02.08.2019 13:30


History, 02.08.2019 13:30



Physics, 02.08.2019 13:30



![\text{Rate}=k[A][B][C]^2](/tpl/images/0287/3858/2c5a0.png)
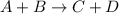
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0287/3858/10aeb.png)
![[A]](/tpl/images/0287/3858/6aa06.png) and
and ![[B]](/tpl/images/0287/3858/db909.png) = concentration of A and B reactant
= concentration of A and B reactant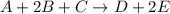
![\text{Rate}=k[A]^1[B]^1[C]^2](/tpl/images/0287/3858/d33f5.png)





