Chemistry, 21.01.2020 01:31, AlaskaAirlines
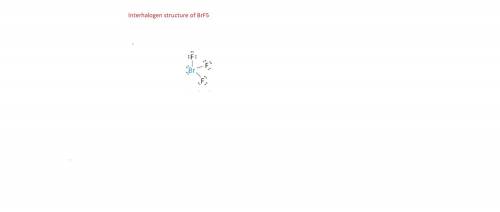
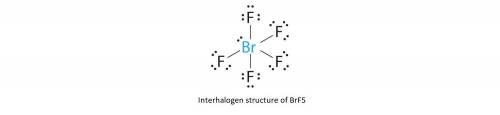
Add lone pairs to these lewis structures of interhalogen compounds brf3 brf5

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3


Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
Do you know the correct answer?
Add lone pairs to these lewis structures of interhalogen compounds brf3 brf5...
Questions in other subjects:

History, 04.07.2019 13:00





History, 04.07.2019 13:00


Mathematics, 04.07.2019 13:00

Mathematics, 04.07.2019 13:00

Biology, 04.07.2019 13:00