
Chemistry, 08.11.2019 02:31, pattydixon6
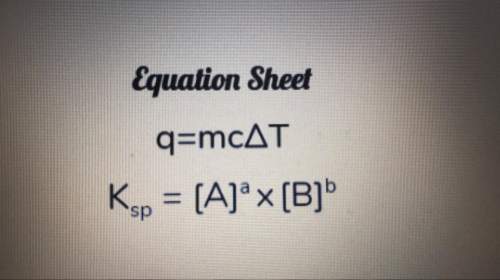
A3.50-g piece of metal is heated, and temperature of the metal changes from 17.0°c to 82.0°c. the amount of heat absorbed is 1435 j. what is the specific heat of the metal? show your work.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 07:30, libbymcvay
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
Do you know the correct answer?
A3.50-g piece of metal is heated, and temperature of the metal changes from 17.0°c to 82.0°c. the am...
Questions in other subjects:









Mathematics, 20.03.2020 09:59






