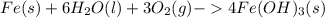One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reacting with oxygen gas in the presence of water to create an oxide layer. iron can form several different oxides, each having its own unique color. red rust is caused by the formation of iron(iii) oxide trihydrate. in the space provided, write the balanced reaction for the formation of fe2o3•3h2o(s). phases are optional.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3



Chemistry, 22.06.2019 06:00, VamPL
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl +4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1
Do you know the correct answer?
One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reac...
Questions in other subjects:

Engineering, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Chemistry, 18.03.2021 14:00


Mathematics, 18.03.2021 14:00



Biology, 18.03.2021 14:00