
Chemistry, 23.09.2019 15:30, babyrocks7300
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
Do you know the correct answer?
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what i...
Questions in other subjects:

Mathematics, 29.08.2019 12:50


Mathematics, 29.08.2019 12:50


History, 29.08.2019 12:50

Biology, 29.08.2019 12:50


Social Studies, 29.08.2019 12:50


Mathematics, 29.08.2019 12:50


 .
.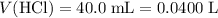 .
.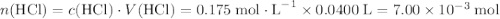 .
.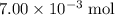 of HCl will neutralize only half that much Ca(OH)₂.
of HCl will neutralize only half that much Ca(OH)₂. .
. .
.



