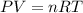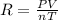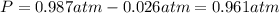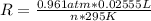Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
Calculate the gas constant, r, if 25.55 ml of hydrogen gas was collected at a barometric pressure of...
Questions in other subjects:

Business, 15.04.2020 20:35



History, 15.04.2020 20:35



Mathematics, 15.04.2020 20:35

Mathematics, 15.04.2020 20:36

Mathematics, 15.04.2020 20:36