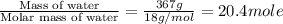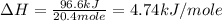Chemistry, 28.09.2019 22:00, emalvidrez5205
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes place in the calorimeter, and the temperature rises to 87°c. the calorimeter contains 367 g of water, which has a specific heat of 4.18 j/(g·°c). calculate the enthalpy change (δh) during this reaction

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
Do you know the correct answer?
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes pl...
Questions in other subjects:


Mathematics, 27.05.2021 20:30

Mathematics, 27.05.2021 20:30

Health, 27.05.2021 20:30

Mathematics, 27.05.2021 20:30

History, 27.05.2021 20:30

Biology, 27.05.2021 20:30


English, 27.05.2021 20:30

Mathematics, 27.05.2021 20:30


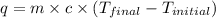
 = specific heat of water =
= specific heat of water = 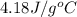
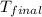 = final temperature of water =
= final temperature of water = 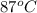
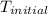 = initial temperature of metal =
= initial temperature of metal = 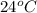
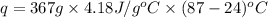
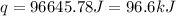
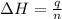
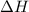 = enthalpy change = ?
= enthalpy change = ?