when 4.33g of o2 and 16.3g of mg react, how many grams of mgo will be formed?


Answers: 1
Similar questions

Chemistry, 22.07.2019 02:01, salam6809
Answers: 1

Chemistry, 26.07.2019 21:00, johnsonkia873
Answers: 1

Chemistry, 11.10.2019 13:30, shauntleaning
Answers: 1
Do you know the correct answer?
Limiting
when 4.33g of o2 and 16.3g of mg react, how many grams of mgo will be formed?
when 4.33g of o2 and 16.3g of mg react, how many grams of mgo will be formed?
Questions in other subjects:




Mathematics, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31


Mathematics, 28.01.2020 07:31




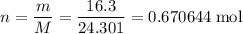 .
. .
. of MgO will be produced if O₂ is in excess.
of MgO will be produced if O₂ is in excess. .
. .
.



