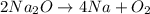Determine the atom inventory for the following equation: 2na2o ➡ 4na + o2. does this equation follow the law of conservation of mass?
na = 2, o = 1; na = 4, o = 1; no, this equation does not follow the law of conservation of mass
na = 4, o = 2; na = 4, o = 2; yes, this equation does follow the law of conservation of mass
na = 4, o = 4; na = 4, o = 2; yes, this equation does follow the law of conservation of mass
na = 2, o = 2; na = 4, o = 2; no, this equation does not follow the law of conservation of mass

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Falconpride4079
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
Do you know the correct answer?
Determine the atom inventory for the following equation: 2na2o ➡ 4na + o2. does this equation follo...
Questions in other subjects:

Mathematics, 10.12.2020 08:30

Mathematics, 10.12.2020 08:30

English, 10.12.2020 08:30



Mathematics, 10.12.2020 08:30