
Chemistry, 28.01.2020 13:40, heyyyyy3922
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 15.51 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 15.46 g of the fuel as well as 0.0817 g of water and 0.1497 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 02:30, kieante01
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
Do you know the correct answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions in other subjects:






Biology, 08.04.2020 04:42





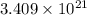
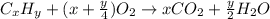
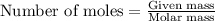
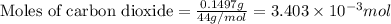
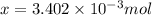
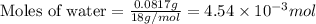
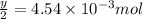
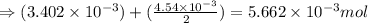
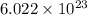 number of molecules.
number of molecules.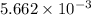 moles of oxygen will contain =
moles of oxygen will contain = 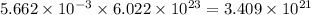 molecules.
molecules.




