
Chemistry, 22.12.2019 12:31, icecreamisgood4u
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic solution, they dissolve: caco3(s) + 2hcl(aq) ➡ cacl2(aq) + h2o(l) + co2(g). how many moles of caco3 can be dissolved in 0.0250 moles of hydrochloric acid?
0.0125 moles
0.0500 moles
2.50 moles
4.50 moles

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
Do you know the correct answer?
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic...
Questions in other subjects:

Mathematics, 18.02.2021 17:50

Mathematics, 18.02.2021 17:50

Mathematics, 18.02.2021 17:50

History, 18.02.2021 17:50




Mathematics, 18.02.2021 17:50

History, 18.02.2021 17:50

Mathematics, 18.02.2021 17:50


![CaCO_3(s)+2HCl(aq)]\rightarrow CaCl_2(aq)+H_2O(l)+CO_2(g)](/tpl/images/0429/7730/2251f.png)
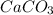
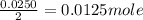 of
of 



