

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, brandon1748
Two liquids are shaken together in a test tube to produce a mixture that quickly separates into two layers. which of the following best describes the behavior of the above pair of substances? soluble insoluble miscible immiscible
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2
Do you know the correct answer?
When 5.12 g of naoh were dissolved in 51.55 g water in a calorimeter at
24.5°c, the temp...
24.5°c, the temp...
Questions in other subjects:


Mathematics, 30.01.2021 02:30



English, 30.01.2021 02:30

Physics, 30.01.2021 02:30

Social Studies, 30.01.2021 02:30


Social Studies, 30.01.2021 02:30

Mathematics, 30.01.2021 02:30


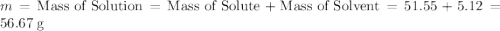 .
.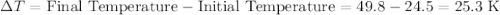 .
.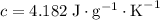 .
.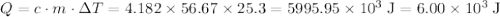 .
.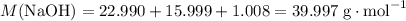 .
.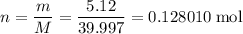 .
.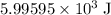 of energy.
of energy.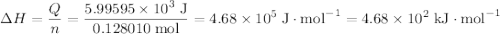 .
.



