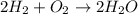Chemistry, 29.01.2020 08:45, swelch2010
Which of the following is true for balancing equations? a. the number of products should be equal to the number of reactants. b. the properties of products should be the same as the properties of reactants. c. there must be as equal number of compounds on both sides of the equation. d. there must be as equal number of atoms of each element on both sides of the equation.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1



Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
Do you know the correct answer?
Which of the following is true for balancing equations? a. the number of products should be equal t...
Questions in other subjects:





Physics, 04.06.2020 20:00





Computers and Technology, 04.06.2020 20:00