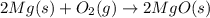Chemistry, 20.11.2019 16:31, reganleigh00
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
a) mg(s) + o2(g) → mgo(s)
b) 2mg(s) + o2(g) → 2mgo(s)
c) 2mgo(s) → 2mg(s) + o2(g)
d) 2mg(s) → 2mgo(s) + o2(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
Do you know the correct answer?
The oxidation of magnesium to form magnesium oxide is shown by which balanced chemical equation?
Questions in other subjects:

Mathematics, 10.12.2020 15:30

Health, 10.12.2020 15:30

Biology, 10.12.2020 15:30


Mathematics, 10.12.2020 15:30

Arts, 10.12.2020 15:30



Medicine, 10.12.2020 15:30