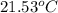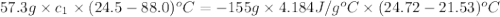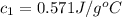Apiece of metal weighing 57.3 g is heated to a temperature of 88.0°c and is then immersed in 155 g of water at a temperature of 21.53°c. after equilibration the temperature is 24.72°c. if ch2o = 4.184 j/g°c, what is cmetal?
a) .370 j/g°c
b) .164 j/g°c
c) 1.00 j/g°c
d) 2.11 j/g°c
e) .571 j/g°c

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
Do you know the correct answer?
Apiece of metal weighing 57.3 g is heated to a temperature of 88.0°c and is then immersed in 155 g o...
Questions in other subjects:




Computers and Technology, 18.01.2020 01:31







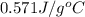 .
.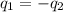
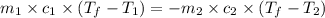
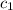 = specific heat of metal = ?
= specific heat of metal = ?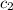 = specific heat of water =
= specific heat of water = 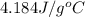
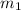 = mass of metal = 57.3 g
= mass of metal = 57.3 g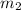 = mass of water = 155 g
= mass of water = 155 g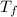 = final temperature of water =
= final temperature of water = 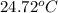
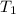 = initial temperature of metal =
= initial temperature of metal = 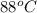
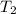 = initial temperature of water =
= initial temperature of water =