
Chemistry, 05.01.2020 14:31, twirlergirl800
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1800m naoh solution. calculate the volume of naoh solution the student will need to add to reach the equivalence point. be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
Do you know the correct answer?
Achemistry student weighs out 0.0634g of formic acid hcho2 into a 250.ml volumetric flask and dilute...
Questions in other subjects:








Mathematics, 15.08.2020 01:01



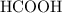 :
: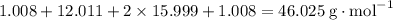 .
.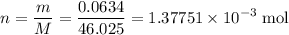 .
.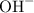 ion to neutralize each carbonyl group
ion to neutralize each carbonyl group 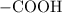 .
.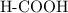 molecule. Each formula unit of NaOH supplies one
molecule. Each formula unit of NaOH supplies one 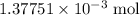 of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.
of formic acid in the volumetric flask. It will take the same number of NaOH formula units to reach the equivalence point.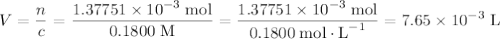 .
.



