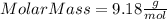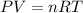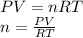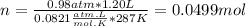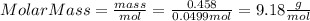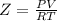Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k and 0.980 atm. show your work. part 2. if this sample was placed under extreme pressure, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, tristen2001
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
Do you know the correct answer?
Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k an...
Questions in other subjects:


Chemistry, 23.04.2020 18:29

Mathematics, 23.04.2020 18:29

History, 23.04.2020 18:29

History, 23.04.2020 18:29


English, 23.04.2020 18:29

History, 23.04.2020 18:30

English, 23.04.2020 18:30

History, 23.04.2020 18:30