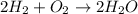Chemistry, 03.02.2020 11:47, nikoidurrant
Hydrogen and oxygen gas combine to form water. if 1.00g of hydrogen and 1.00g of oxygen are reacted, what is the theoretical yield of water in grams?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 16:00, Karinaccccc
The electron configuration for chromium is 1s22s22p63s23p63d54s1 instead of 1s22s22p63s23p63d44s1. the configuration is an exception to the pauli exclusion principle heisenberg uncertainty principle aufbau principle schrödinger equation
Answers: 3

Chemistry, 23.06.2019 21:50, lanipooh01
41. we want to mark off a thermometer in both celsius and fahrenheit temperatures. on the celsius scale, the lowest temperature mark is at and the highest temperature mark is at 50 °c. what are the equivalent fahrenheit temperatures? petrucci, ralph h.. general chemistry (p. 28). pearson education. kindle edition.
Answers: 3

Chemistry, 23.06.2019 22:20, devoncruz23
For a reaction delta h= -286 kj. when is the reaction spontaneous a: when t delta s = 2(-286 kj) b: when t delta s= -286 kj c: when t delta s < -286 kj d: when t delta s > 0
Answers: 3
Do you know the correct answer?
Hydrogen and oxygen gas combine to form water. if 1.00g of hydrogen and 1.00g of oxygen are reacted,...
Questions in other subjects:



History, 25.02.2021 23:50


Mathematics, 25.02.2021 23:50





Mathematics, 25.02.2021 23:50