Chemistry, 03.02.2020 08:57, tasnimabdallah971
Energy and specific heat
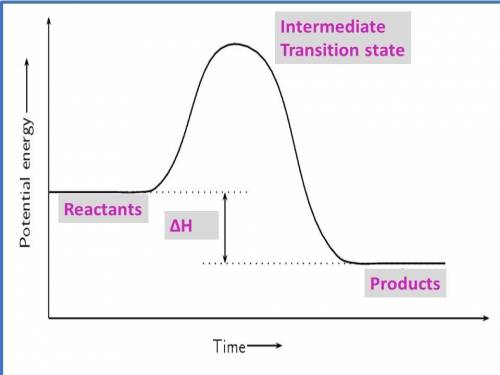
1. draw a graph of an exothermic reaction. label reactants, products and ∆h.
2. calculate the amount of energy required to raise the temperature of 3.00g of gold from 45.9 to 93.0°c.
3. 1.70g of a silvery metal requires 1000.j of energy to change its temp from 298k to 2749k. is the metal pure silver?


Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1
Do you know the correct answer?
Energy and specific heat
1. draw a graph of an exothermic reaction. label reactants, prod...
1. draw a graph of an exothermic reaction. label reactants, prod...
Questions in other subjects:

English, 08.10.2019 01:30


Mathematics, 08.10.2019 01:30




Mathematics, 08.10.2019 01:30


Biology, 08.10.2019 01:30