
Chemistry, 02.10.2019 01:00, divagothboi
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statements is correct about the δh for the products?
a. it is less than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
b. it is less than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.
c. it is greater than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
d. it is greater than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
Do you know the correct answer?
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statement...
Questions in other subjects:


Biology, 25.07.2019 11:30


Mathematics, 25.07.2019 11:30


English, 25.07.2019 11:30





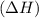 comes out to be positive.
comes out to be positive.



