Chemistry, 28.01.2020 07:31, kingdevin16
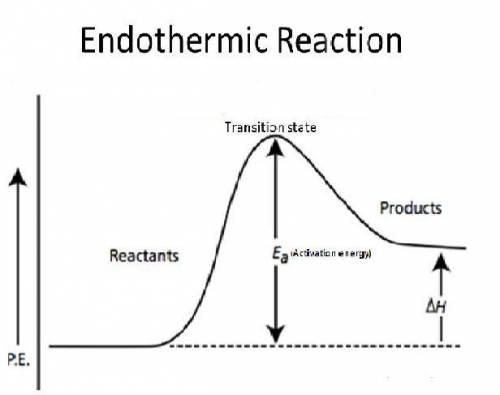
Why does every chemical reaction require a certain amount of activation energy?
a.
energy is released when the reactants begin to react.
b.
energy lost to the environment during the reaction must be replaced.
c.
forming the activated complex requires energy.
d.
the products have more potential energy than the activated complex.
e.
the reactants have less potential energy than the products.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
Do you know the correct answer?
Why does every chemical reaction require a certain amount of activation energy?
a.
en...
a.
en...
Questions in other subjects:



Mathematics, 31.08.2019 20:50


Mathematics, 31.08.2019 20:50

Mathematics, 31.08.2019 20:50