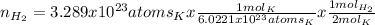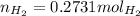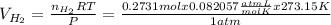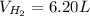Chemistry, 01.11.2019 05:31, lkarroum3733
If 3.289 x 1023 atoms of potassium react with excess water, how many liters of hydrogen gas would be produced at stp? (hint: watch significant figures and rounding.)
2 k + 2 h2o à 2 koh + h2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, Countryqueen525
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
Do you know the correct answer?
If 3.289 x 1023 atoms of potassium react with excess water, how many liters of hydrogen gas would be...
Questions in other subjects:


Mathematics, 18.12.2020 19:40

English, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40


Mathematics, 18.12.2020 19:40


Law, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40

English, 18.12.2020 19:40