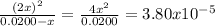The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, the equilibrium constant kc for the reaction is 3.80 × 10−5. suppose you start with 0.0456 mol of i2 in a 2.28−l flask at 1000 k. what are the concentrations of the gases at equilibrium? what is the equilibrium concentration of i2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1


Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
Do you know the correct answer?
The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, th...
Questions in other subjects:


History, 24.06.2019 22:00

Mathematics, 24.06.2019 22:00


Mathematics, 24.06.2019 22:00





English, 24.06.2019 22:00


![\frac{[I]^{2} }{[I_{2} ]}](/tpl/images/0472/7611/f811c.png)
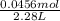 → [I₂]₀ = 0.0200 M
→ [I₂]₀ = 0.0200 M![\frac{[I]^{2} }{[I_{2} ]} = \frac{(2x)^{2} }{0.0200 - x} = 3.80x10^{-5}](/tpl/images/0472/7611/3afcc.png)