
Chemistry, 30.09.2019 08:30, winterblanco
Read the given equation.
2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 6.30 liters of h2 gas were produced at stp?
a. 10.3 grams
b. 12.9 grams
c. 14.7 grams
d.15.2 grams

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Averybeam300
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
Do you know the correct answer?
Read the given equation.
2na + 2h2o → 2naoh + h2
during a laboratory experim...
2na + 2h2o → 2naoh + h2
during a laboratory experim...
Questions in other subjects:

Mathematics, 17.12.2020 17:50

Social Studies, 17.12.2020 17:50


Advanced Placement (AP), 17.12.2020 17:50

Mathematics, 17.12.2020 17:50


Computers and Technology, 17.12.2020 17:50


Mathematics, 17.12.2020 17:50

English, 17.12.2020 17:50


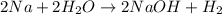
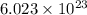 of particles and weighs equal to the molecular mass.
of particles and weighs equal to the molecular mass. is produced by 46 gram of sodium metal
is produced by 46 gram of sodium metal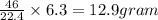 of sodium metal
of sodium metal



