
Chemistry, 06.10.2019 07:11, kberly3750ovgw6f
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be formed by reacting 4 atoms of iron (fe) with 3 molecules of oxygen gas (o2). if 12 atoms of iron are reacted with 6 molecules of oxygen gas, which is the limiting reactant and how many atoms or molecules will be left over? 4fe + 3o2 -> 2fe2o3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
Do you know the correct answer?
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be for...
Questions in other subjects:





Mathematics, 27.09.2019 16:10




History, 27.09.2019 16:10


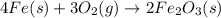
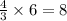 atoms of iron.
atoms of iron.




