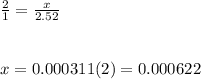Chemistry, 04.02.2020 14:57, alexis3744
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out 28.mg of oxalic acid h2c2o4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.ml of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used 28.4ml of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
Do you know the correct answer?
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs ou...
Questions in other subjects:









Physics, 09.11.2020 22:00