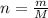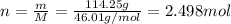Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, chhimmidemg
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
Do you know the correct answer?
The molar mass of no2 is 46.01 g/mol. how many moles of no2 are present in 114.95g?...
Questions in other subjects:



Mathematics, 24.08.2021 20:30

English, 24.08.2021 20:30

Mathematics, 24.08.2021 20:30

English, 24.08.2021 20:30


Arts, 24.08.2021 20:30

Mathematics, 24.08.2021 20:30

Mathematics, 24.08.2021 20:30