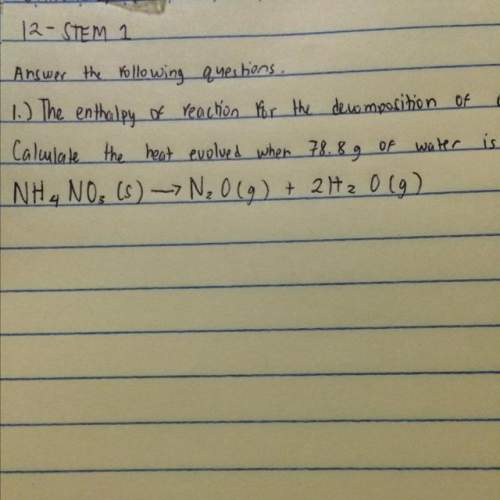Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
Do you know the correct answer?
The enthalpy of reaction for the decomposition of ammonium nitrate, nh4no3 is -77.4 kj mol^-1. calcu...
Questions in other subjects:

Mathematics, 17.05.2021 21:50

English, 17.05.2021 21:50


Physics, 17.05.2021 21:50

Mathematics, 17.05.2021 21:50

Mathematics, 17.05.2021 21:50


Business, 17.05.2021 21:50


Geography, 17.05.2021 21:50