
Chemistry, 29.01.2020 22:03, EinsteinBro
Calculate the volume of a 1.420 m naoh solution required to titrate 34.55 ml of a 1.500 m h3po4 solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1
Do you know the correct answer?
Calculate the volume of a 1.420 m naoh solution required to titrate 34.55 ml of a 1.500 m h3po4 solu...
Questions in other subjects:

Mathematics, 14.12.2020 20:10

Computers and Technology, 14.12.2020 20:10




Advanced Placement (AP), 14.12.2020 20:10

Computers and Technology, 14.12.2020 20:10



Biology, 14.12.2020 20:10


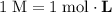 .
.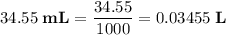 .
.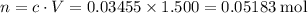 .
.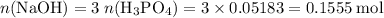 .
.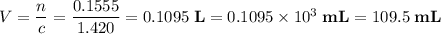 .
.



