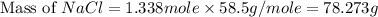Chemistry, 31.01.2020 23:04, sjjarvis53211
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 280. k and 1.50 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, angelrenee2000
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent. true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight. a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
Do you know the correct answer?
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use...
Questions in other subjects:


History, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00

Chemistry, 13.12.2021 14:00

Mathematics, 13.12.2021 14:00



Mathematics, 13.12.2021 14:00


Mathematics, 13.12.2021 14:00


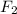 gas by using ideal gas equation.
gas by using ideal gas equation.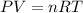
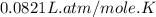
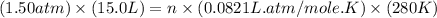
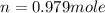
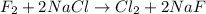
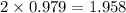 moles of NaCl
moles of NaCl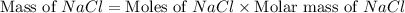
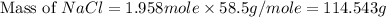
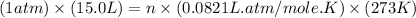
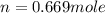
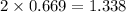 moles of NaCl
moles of NaCl