
Chemistry, 14.12.2019 04:31, araminaara691
The freezing of methane is an exothermic change. what best describes the temperature conditions that are likely to make this a spontaneous change? any temperature, because entropy increases during freezing. any temperature, because entropy decreases during freezing. low temperature only, because entropy decreases during freezing. high temperature only, because entropy increases during freezing.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
Do you know the correct answer?
The freezing of methane is an exothermic change. what best describes the temperature conditions that...
Questions in other subjects:


History, 03.11.2020 17:20

English, 03.11.2020 17:20

Biology, 03.11.2020 17:20

Mathematics, 03.11.2020 17:20




Mathematics, 03.11.2020 17:20

English, 03.11.2020 17:20


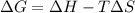
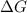 = change in Gibb's free energy
= change in Gibb's free energy 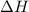 = change in enthalpy
= change in enthalpy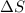 = change in entropy
= change in entropy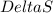 is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the
is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the ![-ve=-ve-[T(-ve)]\\\\-ve=-ve+T](/tpl/images/0418/2588/a4550.png)
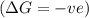 , the temperature must be low.
, the temperature must be low.



